Electrode Cement Material Is Also A Matter Of Learning.
Electrode cement is one of the important auxiliary functional materials in lithium-ion batteries. Although it has no capacity, it has a small proportion in the battery, but it is the main source of mechanical properties of the whole electrode. The electrochemical performance of the process and battery has an important impact. In addition to the bonding properties of general adhesives, lithium ion battery electrode binder materials also need to withstand the swelling and corrosion of the electrolyte, as well as the electrochemical corrosion during charging and discharging, the working voltage of the electrode It is stable in the range, so there are not many polymer materials that can be used as electrode binders for lithium ion batteries.
There are three main types of lithium ion battery binders that are widely used today: polyvinylidene fluoride (PVDF), styrene butadiene rubber (SBR) emulsion and carboxymethyl cellulose (CMC), in addition to polyacrylic acid (PAA), polypropylene. Aqueous binders containing nitrile (PAN) and polyacrylate as main components also occupy a certain market. PVDF is the first widely used lithium ion battery electrode binder, which has strong electrochemical corrosion resistance and can be applied to positive electrode materials. However, PVDF requires N-methylpyrrolidone (NMP) as a solvent, and the cost of recovering the solvent is high, which causes some pollution to the environment. Therefore, aqueous SBR and CMC binders are usually used in a graphite anode having a relatively low potential. Domestically used PVDF adhesives are mainly supplied by European and Japanese manufacturers, while water-based adhesives SBR and CMC are basically controlled by Japanese manufacturers.
As the country's requirements for environmental protection and battery energy density continue to increase, many new types of adhesives are beginning to emerge. On the one hand, in response to increasingly stringent environmental policies, adhesive manufacturers are stepping up the development of water-based adhesives for cathode materials. On the other hand, in order to further increase the energy density of lithium-ion batteries, battery manufacturers have gradually begun to apply high-nickel cathode materials and silicon-carbon anode materials. Although the silicon material has an ultra-high theoretical specific capacity (4200 mA·h/g, calculated as Li22Si5) and a lower discharge potential (about 370 mV, vs. Li/Li+), it is a highly promising negative electrode material. However, the volume change of the silicon material in the process of lithium insertion/delithiation is as high as 300%, and even after being compounded with the carbon material, the volume change is still remarkable. The huge volume change causes the active material particles to break and slip, and eventually leads to electrode powdering, capacity reduction, and cycle life shortening. In order to cope with the volume change of silicon-carbon anode material during charge and discharge, a variety of new lithium-ion battery electrode binders have been developed, including PVDF modified binder, CMC cross-linking modified binder, and polyacryl-modified binder. , sodium alginate binder, conductive adhesive, etc.
Electrode binders involve many performance parameters, including basic physicochemical properties and mechanical properties of the binder, as well as adhesion properties, rheological properties, and electrochemical properties. In addition to this, some of the characteristics of the electrode paste and the electrode are mainly determined by the characteristics of the binder.
Bond properties and test methods
Requirements and characteristics of lithium ion electrode battery binder
Although the lithium ion battery electrode binder has a small specific gravity in the battery and does not have a capacity by itself, the homogenization process of the electrode slurry, the maximum coating thickness of the electrode, the flexibility of the electrode, the energy density of the battery, and the cycle life. Other aspects have important implications. The ideal lithium ion battery electrode binder should have the following properties:
1 good solubility, fast dissolution rate and high solubility;
2 Solvent is safe, environmentally friendly, non-toxic, and water is the best solvent;
3 large molecular weight, small amount of binder;
4 moderate viscosity, easy to homogenize and maintain slurry stability;
5 strong adhesive force, the prepared electrode peeling strength is large;
6 electrochemical properties are stable, no redox reaction occurs in the working voltage;
7 resistant to electrolyte corrosion;
8 has a certain flexibility, can withstand the bending of the electrode and the volume change of the active material particles;
9 conductivity and lithium ion conductivity is good;
10 Wide range of sources and low cost.
However, in fact, the ideal binder does not exist, and various characteristics cannot be obtained. The actual binder can only satisfy some properties. Therefore, in practical applications, different binders or a plurality of binders are often used in the positive and negative electrodes to exert the characteristics of various binders.
Simple bonding model
At present, there are many different theories and hypotheses about the mechanism of action of binders in lithium-ion battery electrodes, such as point bonding models and surface bonding models. Among these theories, the model proposed by HERNANDEZ et al. can be used to roughly describe the role of the binder in lithium ion batteries, and provide a reference for the characterization method of the binder. HERNANDEZ believes that the binder between the active material particles in the electrode and the active material particles and the binder at the interface of the current collector play a major role in bearing and transmitting the stress on the electrode. The mechanical properties of the electrode depend on the adhesion of the binder to the active material, the adhesion of the binder to the current collector, and the bulk strength of the binder. When the stress is greater than the minimum of the three The electrode will be destroyed. Among them, the adhesion of the adhesive can be estimated using the formula (1).
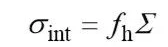 (1)
(1)
ndicates the overall bonding strength of the bonding interface; δint indicates the density of the bonding points on both sides of the bonding interface; and fh is the bonding force of a single bonding point. Based on this model, it can be considered that there are mainly three interactions among the lithium ion battery electrodes: the interaction between the binder and the active material particles, the interaction between the binder and the current collector, and the binder molecules and The interaction between the molecules of the binder. By measuring the values of these three and combining the total area of the bonding interface, the mechanical strength of the electrode can be estimated. Therefore, the bonding property of the bonding material can be divided into the following three parts: the mechanical properties of the bonding agent body, the bonding strength of the bonding agent to the current collector, and the bonding strength of the bonding agent to the active material.
Tensile properties
The bulk strength of the electrode binder is mainly considered to be its tensile properties, which can be described by parameters such as tensile strength, elongation at break, modulus of elasticity, and elastic limit. The binder material can be classified into a brittle binder and a ductile binder according to the difference in the stretching curve. Brittle adhesives have relatively high tensile strength but little elongation at break, and CMC belongs to this category. The tensile strength of the ductile cement is relatively low, but the elongation at break is large, and SBR and PVDF belong to this category. The tensile properties of the binder restrict the ability of the entire electrode to withstand external forces. If the tensile strength of the polymer is too low and the electrode is subjected to an external force, the failure of the bond will first occur from the bulk failure of the binder material, which is not conducive to the overall mechanics of the electrode. Performance improvement. The elastic limit of a polymeric material is the maximum elongation at which the polymer can return to its original shape after stretching. The elastic limit and elongation at break have a certain influence on the reversible specific capacity and capacity retention of the electrode, especially the silicon negative electrode and the silicon carbon negative electrode with obvious volume expansion. The greater the elastic limit and elongation at break of the binder, the greater the deformation that the binder can withstand, and the more stable it is to maintain battery performance.
The tensile properties of the polymer material depend mainly on the type of polymer and its molecular weight. For the same polymer, the higher the molecular weight, the higher the tensile strength. In addition, the topological structure, regularity, crystallinity of the polymer, and molecular weight distribution of the polymer chain also affect its tensile properties. Although increasing the molecular weight of the polymer improves its mechanical properties, it also improves the stability of the binder and the ability to withstand electrolyte corrosion, but it leads to a decrease in the solubility. Therefore, it is necessary to comprehensively consider the performance of various aspects of the lithium ion battery binder, and adjust the molecular weight, molecular chain topology, regularity, crystallinity and the like of the polymer. The national standard GB/T 1040—2016 specifies the test method for the tensile properties of the polymer. In the test, it is recommended to use a molding and extrusion method to prepare a sample, or to form a sheet or a film by a solution method to perform a tensile test. Considering the binder material that is swollen by the electrolyte, the tensile properties may change. It is recommended to test the tensile properties before and after swelling by the electrolyte.
Bond strength
Adhesive strength is the core performance of the bond and can be expressed using tensile strength and tensile shear strength of the butt joint. The tensile strength of the butt joint refers to the maximum tensile stress that the adhesive joint's butt joint specimen can withstand when stretched. The tensile shear strength refers to the maximum shear stress that the bonding surface can withstand when the single lap joint to which the adhesive is bonded is stretched in a direction parallel to the bonding surface. When the active material particles expand due to lithium intercalation, or the electrodes are subjected to external bending, causing slippage and separation between the particles, the stress on the bonding surface can be decomposed into tensile stress and shear stress. Corresponding to the tensile strength and tensile shear strength of the butt joint. The greater the tensile strength and tensile shear strength of the butt-jointed joint of the cement, the stronger the ability to withstand separation and slip between the particles. The tensile strength and tensile shear strength of the butt joints can be tested according to GB/T6329-1996 and GB/T7124-2008, respectively. According to these two standards, it is necessary to prepare a block material of a certain shape and size, and it is required that the tensile strength of the bulk material is greater than the adhesive strength of the adhesive, and the active material used for the lithium ion battery is usually a powder material, These two tests are more suitable for the characterization of the bond strength of the binder to the material of the bulk sample such as current collector or pure silicon.
Peel strength
Peeling strength is the amount of force that can be withstood by the bonding edge per unit length when the external stress concentrates on the edge of the bonding point and the bonding surface is gradually peeled off. It is expressed in kN/m, and N/cm is also used in practical applications. Indicates that 1 kN/m = 10 N/cm. Different from the adhesion strength test, it is necessary to use a block-shaped adherent preparation sample for testing. The peel strength test can directly use the electrode as a sample, and the preparation method is simpler, and can better reflect the true bonding state of the electrode. Peel strength test can be tested by using the floating roll method according to GB/T 7122-1996, or by 180 ° peel test according to the method provided in GB/T2790-1995. Both test methods require the electrode to be attached to a rigid substrate and stripped with an adhesive tape. The test method provided by GB/T7122-1996 requires special fixtures for testing, and the method of GB/T2790-1995 has no special requirements for this, and the latter is recommended. The adhesive tape used in the test should be an ideal flexible material that cannot be irreversibly deformed during the test. At the same time, the adhesive force of the adhesive tape must be strong enough, and the width of the adhesive tape should be equal to or less than the width of the electrode, so that the peeling process occurs inside the active material coating or between the coating and the current collector, otherwise the test data is invalid. . It should be noted that in the test method provided in GB/T2790-1995, the rate and distance of separation between the collets of the test machine are twice the rate and distance of the peeling edge movement. As a result of the peeling test, if the peeling process occurs inside the electrode coating, it indicates that the bonding effect of the bonding agent on the current collector is stronger than that of the bonding agent to the active material, and the measured data is the bonding agent to the active material. Peel strength; if the stripping process occurs between the electrode coating and the current collector, it indicates that the bonding effect of the binder on the active material is stronger than that of the binder on the current collector, and the measured data is the binder to the current collector. Peel strength; if the stripping process occurs between the adhesive tape and the electrode, it indicates that the selected adhesive tape has insufficient adhesion and the test data is invalid. The peel strength can be taken as the average value of the peel strength in the range of 25 to 125 mm after the peeling process, and the maximum and minimum values of the peel strength in the process are recorded at the same time.
Basic physical and chemical properties of the binder
The basic physicochemical properties of the binder include solid content, density, viscosity, pH and other parameters. It is recommended to directly test the relevant national standards for adhesives. Solid content is one of the basic product parameters of liquid binders. This parameter is required to calculate the amount of binder applied before homogenization. The solid content is also referred to as the “non-volatile content” in the national standard, and refers to the ratio of the mass of the liquid adhesive before and after drying under certain conditions. GB/T2793—1995 stipulates that the non-volatile content of the adhesive should be measured by using a forced air oven to dry the adhesive. The test results retain 3 effective figures. Density is another basic physical parameter of liquid binder. After combining the solid content parameters, the amount of binder can be calculated by volume or flow rate. GB/T13354-1992 It is recommended that the density of liquid adhesive be measured using a 37mL weight cup. This test method is simple and easy to apply, especially suitable for liquid adhesives with high viscosity. Viscosity is one of the important process performance parameters of the binder. If the viscosity of the binder is too small, the viscosity of the prepared slurry is also small, resulting in excessive fluidity of the slurry and easy sedimentation, poor storage stability, and additional thickener added; however, if the viscosity of the adhesive Too large, it is not conducive to the dispersion of active materials and conductive agents. In addition to process properties, the viscosity of the binder can also reflect the molecular weight. When the other conditions are the same, the higher the viscosity means the larger the molecular weight and the better the bonding performance. The national standard GB/T2794—2013 stipulates that the viscosity of the adhesive is measured by a single-cylinder rotary viscometer, and the binder solution needs to be defoamed before the test. The viscosity test results retain three significant figures, expressed in Pa·s, but in practice, mPa·s and cP are often used as units, 1 mPa·s = 1 cP. Temperature, concentration, shear rate, solvent and other factors have a great influence on the viscosity measurement results, and should be marked together with the measured viscosity. The pH of the binder is for aqueous binders. The pH of the binder determines the pH of the electrode paste, and different active substances have different adaptability to pH. Therefore, the pH of the binder directly affects whether the binder can be applied to the active material. The national standard GB/T14518-1993 specifies that the pH of the aqueous binder is tested using a glass electrode acidity meter. The temperature at the test is 25 ° C. Before testing, the acidity meter should be calibrated using two standard solutions similar to the pH of the sample to be tested. The test result is accurate to one decimal place. In the test, the water used to dissolve and dilute the binder should use three grades of water, otherwise the accuracy of the test results may be affected.
Rheological properties of the slurry
The rheological properties of the slurry are critical to the coating process. Coating with a slurry that does not meet the required rheological properties may result in wet film flow, uneven electrode thickness, flow marks on the electrode surface, rough surface, etc. Quality issues. Therefore, special attention needs to be paid to the rheological properties of the electrode, especially the viscosity, flow resistance and self-leveling properties of the slurry. The viscosity can be tested according to the test method of the viscosity of the adhesive, and the equipment of the appropriate range is selected. The flow resistance of the slurry is the ability of the slurry to remain in its original position after application without flowing. It can be tested according to the method provided in GB/T 31113-2014. The test method may be a glue applicator method or a squeegee method, that is, using a plurality of wet slurry strips or coating a large area of slurry on a flat test plate, and after standing for a certain period of time in the test environment, the test slurry is tested. The degree of drooping, the distance from the sag indicates the flow resistance of the slurry, and the smaller the sag distance, the better the flow resistance. Self-leveling performance is the performance of the wet film surface after the coated wet film is parked at a specified temperature for a period of time with only gravity and no additional pressure. It can be provided in accordance with GB/T 33403-2016. The method is tested.
Electrode and electrode coating
In addition to affecting the rheological properties of the slurry, the electrode binder also determines many characteristics of the electrode, such as the adhesion of the electrode coating, the flexibility of the electrode, the surface hardness, and the solvent resistance. The adhesion test of the electrode coating can be carried out according to GB/T1720-1979. Using a sharp needle tip, a round rolling line is drawn on the surface of the coating under a certain pressure, and the adhesion of the coating is graded according to the degree of peeling of the coating. . The results of the adhesion test are similar to those of the peel strength, but are more intuitive. The method of testing the hardness of the paint film provided by GB/T 6739-2006 can be used to test the surface hardness of the electrode coating. A pencil of a prescribed size, shape, and hardness was used to pass the surface of the coating to test the maximum hardness of the pencil that was scratched without scratching the surface hardness of the coating. For the electrode, flexibility is primarily concerned with the minimum radius of curvature that the electrode can withstand when bending, ie the radius of the thinnest shaft that the electrode can use during winding. The flexibility test method specified in GB/T 1731-1993 is carried out in this way, using a shaft rod of different diameters to be wound, so that the coating does not produce the finest damage such as netting, cracks, peeling and the like after unwinding. The diameter of the shaft indicates the flexibility of the coating. The ability of the electrode to resist electrolyte can be referred to the test method provided by the chemical industry standard "HG/T3857-2006 Insulating paint film oil resistance test method". Take the dried electrode, half immersed in the electrolyte, half exposed to the air, and immersed for 24 hours at a certain temperature and then taken out. If the electrode is immersed in the electrolyte and the exposed part of the air remains flat and smooth, without bubbles, wrinkles or falling off, it indicates that the ability to withstand electrolyte is good. In addition to the above mentioned standards, the national standard GB/T 13452.2-2008 also provides a number of methods for testing the thickness of the coating, which can be used to measure the wet film thickness and dry film thickness of the electrode coating, which can help the electrode coating before and after drying. The thickness of the area facilitates control of product quality and adjustment of coating process parameters.
Conductivity
Generally, a conductive agent is added during the electrode fabrication process to enhance the conductivity inside the electrode, and there is no special requirement for the electrode binder to have conductivity. However, if the electrode binder has a certain conductivity, the internal resistance of the battery can be reduced, which is advantageous for the improvement of the battery rate performance. The existing standard "GB/T35494.1-2017 isotropic conductive adhesive test method Part 1: General method" and "HG/T3331-2012 insulating paint film volume resistivity and surface resistivity determination method" provided A method of testing the conductivity of conductive tapes and coatings for reference to conductive adhesives.
Environmental requirements
In addition to the test methods that specify the common properties of the binder, the national standard also specifies the limits and detection methods for volatile organic content (VOC) and soluble heavy metal content in the binder. Volatile organic compounds refer to the total weight of cement products after deducting solid content, moisture, and exempt compounds (acetone, methyl acetate). Currently, there is no VOC limit standard for lithium ion battery binders in the national standard. The detection of soluble heavy metals is mainly for elements such as lead, chromium, cadmium, tellurium, mercury, arsenic, selenium and tellurium which are harmful to the environment. The detection methods are graphite furnace absorption spectroscopy and hydride atomic fluorescence spectrometry.
Development status of lithium ion battery electrode binder
The characteristics of electrode binders are various, covering mechanical properties, rheological properties, bonding properties, electrochemical properties and other aspects of performance, and the performance of various adhesives are different, resulting in its suitable processing. Process and electrode materials also vary. The solubility characteristics and viscosity of the binder mainly affect the processing technology of the electrode. The pH value and electrochemical stability limit the application range of the binder, and the peel strength affects the processing performance and long-term cycle performance of the electrode. Here are some of the basic characteristics of commercialized adhesives and their impact on the range of applications in lithium-ion batteries.
Basic characteristics of commercial adhesives
Although classified as PVDF binder, SBR/CMC binder, PAA binder, etc., the performance of the same kind of binder produced by different manufacturers varies within a certain range, mainly with two grades of binder. Introduce. The positive electrode binder is exemplified by Sowell's Solef 5130 binder.
The main component of Solef 5130 is a copolymer of PVDF. When the amount is 3% (mass fraction), the peeling force of the prepared lithium iron phosphate electrode can reach 0.62 N/cm, and the peeling strength is large. The product has good solubility in NMP, and the viscosity is 8000mPa·s at a concentration of 8% at 25 °C.


